
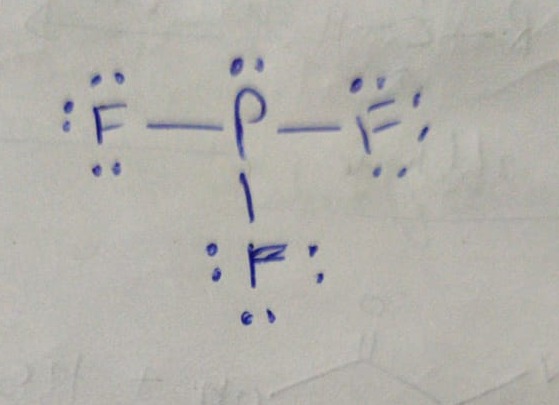
In its molecule, both oxygen atoms have the same electronegativity value, and both atoms share equal ratios of bonded shared electrons, so the overall O 2 molecule turns out to be nonpolar in nature. Pf3 Molecular Geometry - 18 images - ppt chapter 9 molecular structures. So, In PF 3, the phosphorous (P) is a central atom that has one lone pair on it and it is also attached to the three fluorine (F) atoms. The trigonal pyramid geometry is formed when the central atom is attached to three atoms and contains one lone pair. Oxygen is a diatomic nonpolar molecule with a bond angle of 180 degrees. The molecular geometry of PF3 is a Trigonal pyramid. This gives it a VSEPR notation of AX3E and a molecular geometry Show. In the O2 Lewis structure, there is a double bond between two oxygen atoms. PF3 (phosphorus trifluoride) has one phosphorus central atom surrounded by three fluorine atoms and one lone pair of electrons.
It depicts the arrangement of electrons around individual atoms in a molecule.Įlectrons are shown as “dots” or as a line between two atoms when they are bonded. The Lewis structure is a simplified representation of valence shell electrons. Methane contributes to climate change because of its ability to trap heat in the atmosphere. Methane that is discharged into the atmosphere before being burnt, on the other hand, is hazardous to the environment. For VSEPR, it would not affect the general shape of the atom but angles would differ because lone pairs tend to take. But bond polarity of C-S is canceled to each other in the linear. It has a difference in electronegativity values between sulfur and carbon atoms, with sulfur’s pull the electron cloud being slightly higher than carbon’s. Therefore, you would expect 3 single bonds and a lone pair on P. The molecule of carbon disulfide (with linear shape CS2 molecular geometry) is tilted at 180 degree bond angle of S-C-S. What is the molecular notation for PF3 molecule PF3 molecular notation is AX3N1. Fluorine cannot form a double bond since it only needs one more electron to fulfill it's octet and it's unable to form expanded octets. Natural gas, which is largely made up of methane, is the most environmentally friendly fossil fuel. PF3 Molecular geometry is an electronic structural representation of molecules.


 0 kommentar(er)
0 kommentar(er)
